Meeting ISO 11607-1 and Other Regulatory Requirements
Article | June 21, 2019
DuPont™ Tyvek® medical and pharmaceutical packaging styles comply with the materials portion of the ISO 11607-1:2006/Amd.1:2014 Standard. Compliance of Tyvek® to the ISO 11607-1:2006 Standard is supported by DuPont technical information which contains the necessary experimental data. The DuPont™ Tyvek® Compliance to ISO 11607-1:2006/Amd.1:2014 guidebook can be downloaded here.
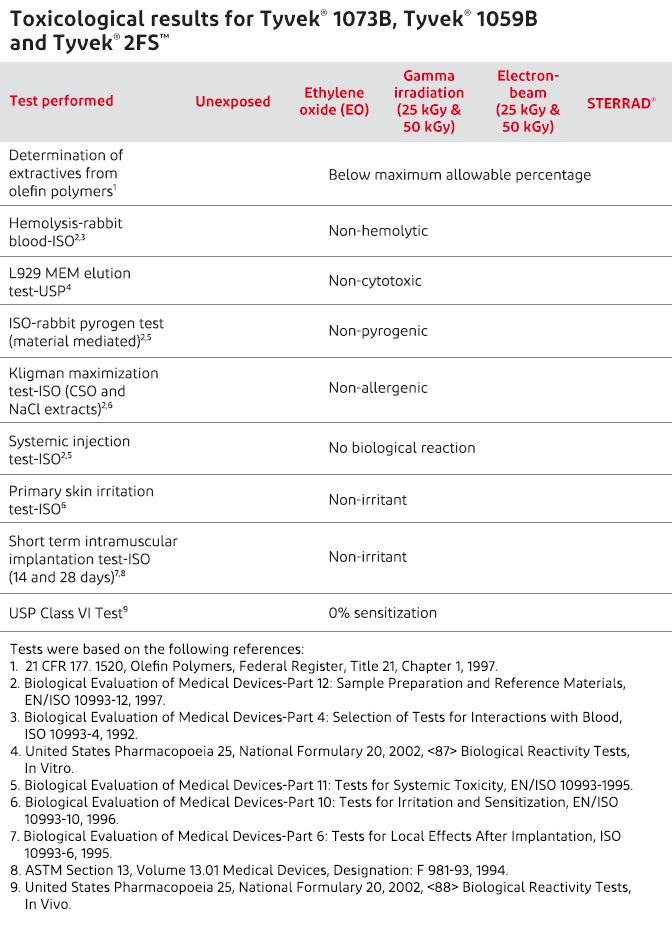
Biocompatibility
Tyvek® medical and pharmaceutical packaging styles meet all the acceptable performance criteria for biocompatibility—even after sterilization—when tested according to ISO 10993 and United States Pharmacopeia (USP).
Food Contact and Pharmacopeia Regulations
Tyvek® medical and pharmaceutical packaging styles meet the extractable or composition requirements of various food contact and pharmacopeia regulations, such as Title 21 of the United States Code of Federal Regulations (21 CFR 177.1520), Commission Regulation (EU) Nº 10/2011 and European Pharmacopoeia, Section 3.1.5 and 3.1.3.
Quality Systems
DuPont manufactures Tyvek® medical and pharmaceutical packaging styles at production facilities located in Richmond, VA, and Luxembourg that are ISO 9001:2015 certified. Both facilities have a Quality Systems Manual, which is a requirement for certification, and strictly adhere to good manufacturing practices, good laboratory practices and quality control measures.