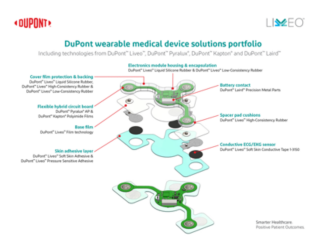
DuPont™ Liveo™
Medical-Grade
Low Consistency Rubbers (LCRs)
DuPont™ Liveo™
Medical-Grade
Low Consistency Rubbers (LCRs)
DuPont™ Liveo™ Low Consistency Rubber (LCR) delivers precision and reliability for complex medical applications. With decades of silicone expertise, Liveo™ offers high-purity, medical-grade materials engineered for mold making, potting, prototyping, supported extrusion, and molding – ensuring consistent flow, excellent biocompatibility and superior performance. Our dedicated healthcare manufacturing and rigorous quality standards help you design safer, more efficient devices with confidence.
Need more information?
What is low-consistency rubber (LCR)?
Low-consistency rubber (LCR) is a silicone or synthetic rubber compound with lower viscosity and softer consistency compared to traditional high-consistency grades. This makes LCR highly flowable and ideal for mold making, prototyping, potting, supported extrusion, and molding. It offers excellent flexibility, chemical resistance and thermal stability, making it suitable for applications including medical components, in which intricate designs and consistent performance are critical.
Our product portfolio
Medical application LCRs with sustained performance for critical non-implant, short-term implant (<30 days), and long-term implant applications (>29 days)*
| Product name | Key characteristics/ certifications | Typical applications | Durometer, Shore A | Cure, components & mix ratio |
Low-temperature cure; 21CFR177.2600, USP-VI, Select ISO10993, European Pharmacopeia, FDA 21 CFR |
Moldmaking, prototyping, encapsulating, drug delivery |
28 |
Platinum-catalyzed; 2-part; 10:1 |
|
Non-filled silicone elastomer gel; Select ISO10993 |
Filling for external prosthetic forms and pressure cushions |
NA |
Platinum-catalyzed; 2-part; 1:1 |
*Use of this material for implantation ≥30 days requires indemnification. It is the user’s responsibility to ensure the safety and efficacy of these materials for all intended uses. While these materials have passed screening tests that are applicable to products intended for implantation, DuPont makes no representation concerning the suitability of these products for implantation applications in the human body.
LCR applications & uses
Our low-consistency rubber elastomers are ideal for a range of specialized medical applications:
- Intricate designs and close-tolerance parts; can be used for spacers, sheets, housing or protection
- Filling material for external form prostheses and pressure cushions
- Precision medical device encapsulating and moldmaking
- General prototyping, molding and fabrication of medical device components
- As a drug matrix for controlled release drug delivery systems
LCR benefits & advantages
Advanced medical-grade Liveo™ LCR materials provide attributes to address specific needs and applications:
- Qualified to meet or exceed test requirements of select U.S. Pharmacopeia Class VI, European Pharmacopoeia (Ph. Eur.), ISO 10993-1, FDA 21 CFR
- Platinum-catalyzed formulations that deliver optimal cohesion and softness for biomedical needs
- Biocompatibility
- Easily processable two-part systems
- Room-temperature and heat-accelerable cure
- Pigmentability